Open Challenge
Generative-AI for Novel Target Combinations
Who we are
At AION Labs, we are committed to partnering with exceptional founders from diverse backgrounds to build groundbreaking companies. Our unique Venture Studio model fosters cross-industry collaboration by connecting global pharmaceutical leaders with the best AI, computational, biotech, and tech entrepreneurs. Through strategic partnerships, funding, expert mentorship, and unparalleled access to pharma R&D expertise, we empower startups to accelerate their journey from idea to market, enabling the creation and validation of transformative solutions that address the industry’s biggest R&D challenges, ultimately driving better healthcare outcomes for humanity.
What we are looking for
We invite exceptional entrepreneurs, scientists, and technologists in computational biology, bioinformatics, AI-driven drug discovery, target identification, machine learning and related fields, including academic experts to submit proposals for a new startup addressing this challenge.
Multi-functional drug modalities, such as bi-specific antibodies and multi-specific peptides, have demonstrated substantial clinical success in treating complex diseases like cancer and metabolic disorders, and hundreds are currently under development. However, designing these drugs requires moving beyond single-target approaches toward multi-targets molecular discovery.
Traditional target discovery relies on expert-driven hypotheses, literature reviews, academic research, and fragmented experimental data, all generated on a single target with a single drug. We seek for a novel AI-driven platform that can systematically identify, rank, and validate molecular target combinations for multi-specific biologics. Prioritizing hypotheses should be based on disease relevance, biomarker predictability, and on-target adverse reaction risks, in therapeutic areas such as Oncology, Cardiovascular-Kidney-Metabolic (CKM), and Immune-mediated disorders.
Existing examples for rational combination include dual signal modulators and immune cell engagers in cancer, complementary biology for additive therapeutic effect (co-agonist) in Obesity, and modulating multiple molecular targets across an organ network in Cardiovascular diseases.
Who should apply
The Founder role is intended to suit candidates who would like to build and lead a startup or aspire to develop themselves toward an entrepreneurship path.
Candidates for Co-Founder roles are expected to have technology experience and have a certain degree of specialization in one or more relevant cutting-edge technologies or scientific areas including computational biology, bioinformatics, complex disease biology, computer science/machine learning, language models, and encryption methods.
We also welcome teams with at least one scientific or technical founder.
Background
The current drug discovery process faces challenges in effectively managing complex diseases like cancer, cardiovascular-kidney-metabolic (CKM) syndrome, and immune-mediated diseases, which require a multi-targets, network-based approach rather than the traditional single-target focus.
AI is transforming biologic drug design, enabling the discovery of drugs that bind and modulate multiple proteins simultaneously. To fully harness this progress, we must identify the right target combinations for multi-specific biologics. As the combinatorial space of multi-target interventions is vast, we are necessitating computational solutions to optimize targets selection.
Problem statement
Current target combination discovery methods primarily rely on manually generated hypotheses derived from literature, expert insights, and competitive intelligence. This approach is time-consuming, prone to redundancy, and often fails to identify novel target combinations.
Existing AI-driven approaches predominantly focus on single-target discovery or repurposing drugs designed for single targets. However, there is a critical need for a system that can generate novel multi-target hypotheses.
A generative AI system integrating systems biology and network pharmacology can address this gap by predicting effective multi-target interventions, identifying biomarkers, and optimizing therapeutic strategies to improve drug development outcomes.
Current approaches
- Manual hypothesis generation: researchers rely on literature reviews, academic insights, and industry intelligence to identify potential targets. This method is inefficient, limited by existing knowledge, and often leads to redundant efforts.
- Multi-omics based approaches: human genetic studies, transcriptomics, and proteomics data contribute to target discovery. While these methods offer insights into disease mechanisms, they typically focus on single-gene associations rather than multi-target strategies.
- AI for single-target identification: knowledge graph-based AI tools assist in single-target identification but lack the capability to systematically propose and validate multi-target hypotheses.
- Molecular dynamics simulations: models relying on known drug-target interactions for multi-target docking by screening drugs against multiple targets to assess binding affinities. This model is limited by structural data and lacks biological context.
- Drug combinations: models that predict synergies between existing drugs but do not generate novel target hypotheses, limiting their application in early-stage drug discovery.
- Network and systems biology models: Computational models integrating biological networks for virtual patient simulation provide insights into molecular interactions, yet they require extensive manual curation and lack automation in novel target combinations.

Rationale for Multi-Target Approach in complex diseases
Inflammatory Diseases
Inflammatory diseases, including autoimmune disorders such as rheumatoid arthritis, inflammatory bowel disease, and psoriasis, arise from dysregulated immune responses that involve multiple signaling pathways and immune cell types. Traditional treatments often target a single cytokine or receptor, but this approach may be insufficient due to compensatory and immune plasticity mechanisms that sustain inflammation.
A multi-target strategy can:
- Block multiple inflammatory mediators to reduce disease severity and progression.
- Enhance immune regulation by modulating both pro-inflammatory and anti-inflammatory pathways.
- Targeting distinct immune pathways in different tissues, ensuring broader efficacy across diseases with systemic and localized components
- Improve therapeutic efficacy by reducing resistance to monotherapy and broadening patient responsiveness.
Bi-specific antibodies and dual cytokine inhibitors, such as IL-17/IL-23 and TNF/IL-6 inhibitors, have demonstrated promise in inflammatory disease treatment. AI-driven generative models can aid in identifying novel target combinations that optimize immune modulation, leading to more effective and personalized treatment strategies.
CKM Syndrome example
Cardiovascular-Kidney-Metabolic (CKM) syndrome is a systemic disorder characterized by interrelated dysfunctions across the cardiovascular, renal, and metabolic systems. It is driven by shared pathological mechanisms such as insulin resistance, chronic inflammation, dyslipidemia, and endothelial dysfunction, contributing to an increased risk of major adverse health outcomes.
Given its multifactorial nature, targeting multiple receptors simultaneously presents a promising therapeutic approach. Single-target interventions often fail to address the interconnected mechanisms underlying CKM syndrome, whereas multi-target strategies can:
- Improve metabolic control by modulating insulin sensitivity, lipid metabolism, and energy balance.
- Reduce cardiovascular risk by regulating vascular inflammation and endothelial function.
- Preserve kidney function by mitigating fibrosis, oxidative stress, and inflammation.
- Reduce compensatory mechanisms and improve long-term treatment response
Bi-specific peptides and multi-receptor agonists, such as GLP-1/GIP and GLP-1/Glucagon co-agonists, have demonstrated significant benefits in addressing CKM syndrome by engaging multiple pathways simultaneously. The list below includes example of multi-specific drugs in clinical development for metabolic diseases:
- Tirzepatide (GLP-1/GIP receptor agonist) – Developed by Eli Lilly, FDA approved in 2021, enhances insulin secretion and reduces appetite.
- Retatrutide (GLP-1/GIP/Glucagon receptor agonist) – Developed by Eli Lilly, Phase 3, promotes weight loss and glycemic control.
- CagriSema (GLP-1/Amylin receptor agonist) – Developed by Novo Nordisk, Phase 3, reduces appetite and improves metabolic outcomes.
- Mazdutide (GLP-1/Glucagon receptor agonist) – Developed by Eli Lilly and Innovent Biologics, Phase 3, modulates energy balance and glucose metabolism.
Oncology example
Cancer is a heterogeneous disease driven by complex interactions between tumor cells, the immune system, and the tumor microenvironment (TME). Traditional single-target therapies often fail due to tumor adaptability and resistance mechanisms.
Multi-target approaches can enhance treatment efficacy by:
- Targeting multiple tumor cell pathways to prevent resistance.
- Modulating immune checkpoints to enhance anti-tumor immunity.
- Disrupting the TME to inhibit cancer progression and metastasis.
- Target a process and its backup simultaneously.
Bi-specific antibodies such as PD-1/CTLA-4 or HER2/HER3, have demonstrated promise in oncology by leveraging dual mechanisms to simultaneously block tumor growth and enhance immune responses.
AI-driven generative models can optimize these strategies by identifying novel, synergistic target combinations for precision oncology interventions.
The following list includes additional examples of multi-specific drugs currently in clinical development in oncology:
- Zenocutuzumab (HER2/HER3 bispecific) – Developed by Merus, FDA-approved, inhibits HER2/HER3-driven tumor growth.
- Amivantamab (EGFR/MET bispecific) – Developed by Janssen, FDA-approved, targets EGFR and MET in NSCLC.
- Amivantamab (EGFR/cMet bispecific) – Developed by Janssen, FDA-approved, overcomes resistance in EGFR-mutated NSCLC.
- Vudalimab (PD-1/CTLA-4 bispecific) – Developed by Xencor, Phase 2, enhances immune response against tumors.
- Tebotelimab (PD-1/LAG-3 bispecific) – Developed by MacroGenics, Phase 1, boosts T-cell activation and tumor suppression.
The desired solution
This system is expected to be integrated into early discovery pipeline. A generative AI model for target combination hypotheses will enhance multi-target drug discovery, overcoming current limitations and fostering innovation in first-in-class therapeutics.
The integration of AI in multi-target drug discovery will revolutionize the identification of new therapeutic strategies. By systematically analyzing large-scale biomedical data, AI can uncover intricate molecular interactions and propose optimized target combinations that may have been overlooked through traditional methods. This computationally driven approach will enhance the discovery process, enabling development of biologics tailored to specific disease mechanisms while reducing the risk of adverse effects.
In production mode, we expect the user to insert the input data, and the AI system to provide the outputs listed below. The training data will be dependent on the selected methodology and solution technology.
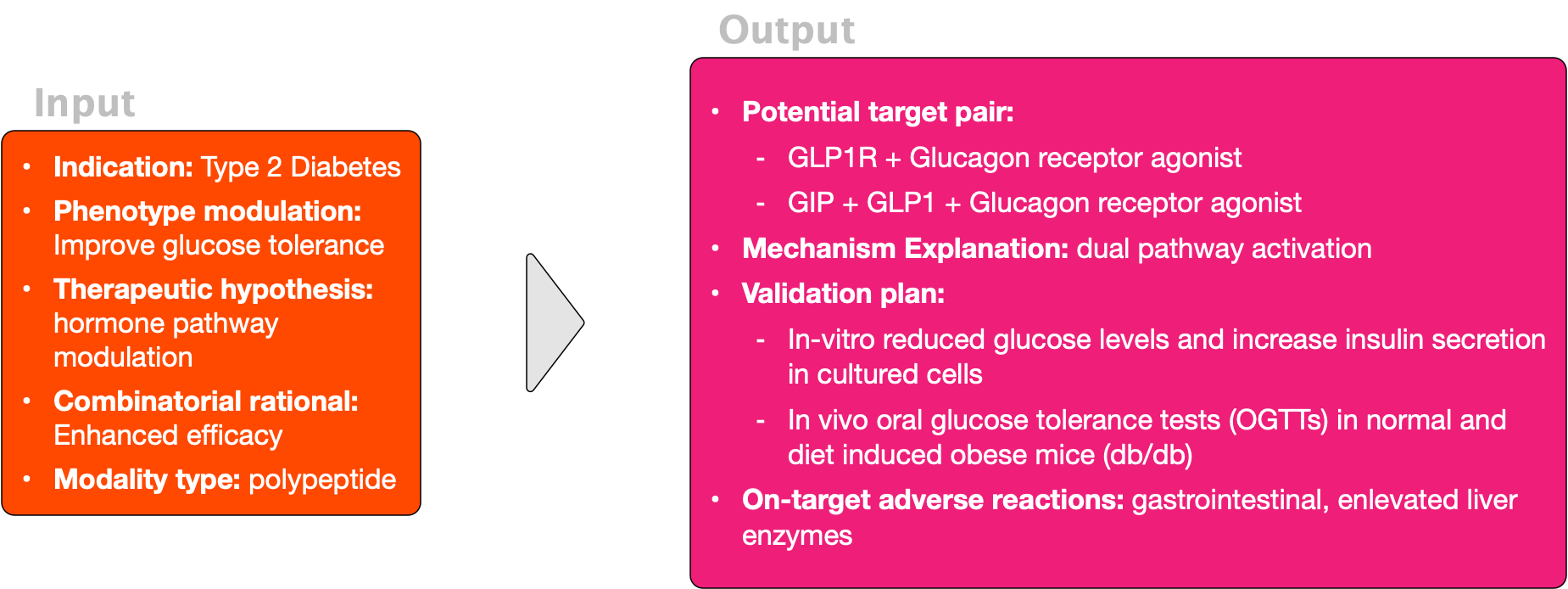
Input:
- Indication and patient population. Definition of the disease and the targeted patients
- The desired modulation of clinical phenotype. Increase or decrease of a measurable clinical readout.
- Rational for the target combination. For example: enhanced activity, cell engagement, cell specificity, resistance, multi organ communication.
- Therapeutic hypothesis indicating what should happen upon engagement of the drug with the target combination.
- Drug modality. Antibody, peptide, mini proteins.
- Other specifications or requirements. For example, protein families.
Outputs:
- Target pair ranking: A prioritized list of potential molecular target combinations aimed at treating the specified indication or modulating the desired phenotype.
- Mechanistic explanation: Provide detailed scientific rationale linking selected molecular target combinations to disease phenotype.
- Biomarker prediction: Identify biomarkers to select patient populations and assess target engagement.
- On-target adverse reactions: Considering potential toxicity and incorporating them into the hypothesis ranking.
- Validation strategy. Propose a functional validation to refine and confirm target combination hypotheses.
An example of an input/output scenario:
References:
Ndumele, C. E., Rangaswami, J., Chow, S. L., Neeland, I. J., Tuttle, K. R., Khan, S. S., … & American Heart Association. (2023). Cardiovascular-kidney-metabolic health: a presidential advisory from the American Heart Association. Circulation, 148(20), 1606-1635.
Brandt, S. J., Müller, T. D., DiMarchi, R. D., Tschöp, M. H., & Stemmer, K. (2018). Peptide‐based multi‐agonists: a new paradigm in metabolic pharmacology. Journal of internal medicine, 284(6), 581-602.
Chelliah, V., & van der Graaf, P. H. (2022). Model‐informed target identification and validation through combining quantitative systems pharmacology with network‐based analysis. CPT: Pharmacometrics & Systems Pharmacology, 11(4), 399.
Dugourd, A., Kuppe, C., Sciacovelli, M., Gjerga, E., Gabor, A., Emdal, K. B., … & Saez‐Rodriguez, J. (2021). Causal integration of multi‐omics data with prior knowledge to generate mechanistic hypotheses. Molecular Systems Biology, 17(1), e9730.
Boran, A. D., & Iyengar, R. (2010). Systems approaches to polypharmacology and drug discovery. Current opinion in drug discovery & development, 13(3), 297.
Dasika, M. S., Burgard, A., & Maranas, C. D. (2006). A computational framework for the topological analysis and targeted disruption of signal transduction networks. Biophysical journal, 91(1), 382-398.
Ruths, D. A., Nakhleh, L., Iyengar, M. S., Reddy, S. A., & Ram, P. T. (2006). Hypothesis generation in signaling networks. Journal of Computational Biology, 13(9), 1546-1557.
Emami, N., & Ferdousi, R. (2024). HormoNet: a deep learning approach for hormone-drug interaction prediction. BMC bioinformatics, 25(1), 87.
Kanehisa, M., Furumichi, M., Sato, Y., Matsuura, Y., & Ishiguro-Watanabe, M. (2024). KEGG: Biological systems database as a model of the real world. Nucleic Acids Research, gkae909.
Rodchenkov, I., Babur, O., Luna, A., Aksoy, B. A., Wong, J. V., Fong, D., … & Sander, C. (2020). Pathway Commons 2019 Update: integration, analysis and exploration of pathway data. Nucleic acids research, 48(D1), D489-D497.
Dönitz, J., & Wingender, E. (2014). EndoNet: an information resource about the intercellular signaling network. BMC systems biology, 8, 1-11.
Von Mering, C., Jensen, L. J., Snel, B., Hooper, S. D., Krupp, M., Foglierini, M., … & Bork, P. (2005). STRING: known and predicted protein–protein associations, integrated and transferred across organisms. Nucleic acids research, 33(suppl_1), D433-D437.
Key Objectives
A successful platform should address the following:
- Target pair ranking: Given an indication, patient population, the desired clinical phenotype modulation and the strategy (synergism/engager/etc.), generate a ranked list of potential target pairs.
- Mechanistic explanation: Provide detailed scientific rationale linking selected molecular target combinations to disease phenotype.
- Biomarker prediction: Identify downstream biomarkers to select patient population and measure target engagement.
- On-target adverse reactions: Considering combinatorial potential toxicity and incorporating them into the hypothesis ranking.
- Validation strategy: Propose a functional validation such as “Lab-in-the-Loop” approach or validation via human datasets to refine and confirm target hypotheses.
The new technology developed by the startup will be tested using selected indications as a proof-of-concept according to the pharma partner’s guidance. Disruptive ideas that go far beyond the current state-of-the-art are particularly encouraged.
What we offer
- A fully funded startup with salaries, services, travel, and access to core facilities in Israel and beyond.
- Founders’ equity in the new startup company.
- An initial investment of at least $1M for the first two years, with working space provided in the AION Labs premises, including wet and computational labs and access to our network of investors for additional funding.
- Mentorship and guidance from pharma, AI, and venture capital experts.
- A dynamic environment in one of the world’s most innovative biomedical ecosystems.
There are flexibility over starting dates, intending to launch the new startup during Q4 2025.
Application & Selection Process
Please apply online at https://career.bio.mx/call/2025-AIL-C08
- As part of the online application procedure, you will be asked to submit: (i) A competitive project proposal addressing the challenge of this call (3-5 pages describing your core hypothesis, scientific rationale, and unique approach to solve the challenge); (ii) Your curriculum vitae including your publication record.
- Deadline for applications: May 5, 2025.
- After a first selection round, candidates will be invited to a five-day innovation workshop in Israel from July 6 to 10, 2025. You will get a chance to receive feedback and guidance from experienced industry mentors. The candidates will jointly work on their project proposals and present them in front of a jury on the final day. Successful candidates will be offered the opportunity to found a startup company at AION Labs in Israel.
For additional information reach out: WorkShop@aionlabs.com